Expandiendo las fronteras de la Ciencia
29 Abr 2024
Cerramos una exitosa nueva edición de nuestras jornadas de difusión “¿Qué Hacemos en el Instituto Leloir?”
Nuestro ya tradicional “QHL” es un evento social destinado a quienes están buscando un lugar donde iniciar o continuar su carrera científica, para que puedan conocer de primera mano cómo trabajamos en la Fundación Instituto Leloir. Durante dos días, unos 55 estudiantes visitaron nuestra sede y otros tantos siguieron las charlas de manera virtual
Ver más
18 Abr 2024
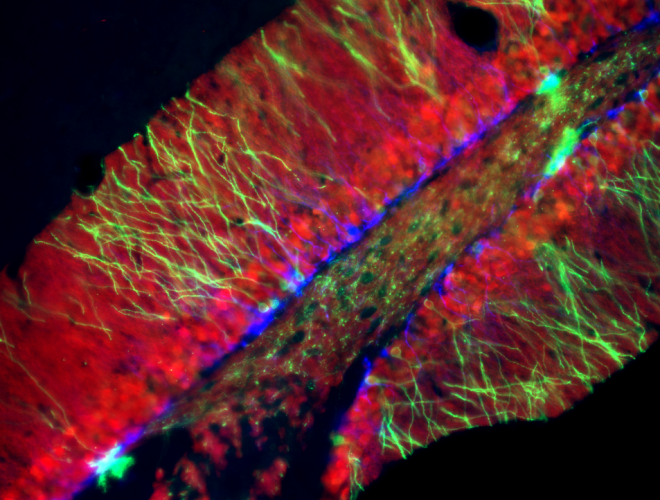
Crean una herramienta que mejora la eficacia de los modelos computacionales de análisis genéticos para identificar personas perdidas
En un paper publicado en Forensic Science International: Genetics, un grupo interdisciplinario liderado por el jefe de nuestro Laboratorio de Biología de Sistemas Integrativa, Ariel Chernomoretz, propone un enfoque novedoso para hacer más eficientes los algoritmos y poder establecer relaciones de parentesco de manera certera, rápida y a gran escala.
Ver más
Áreas de investigación